Every year, thousands of people accidentally take extended-release medications the wrong way - crushing them, splitting them, or taking them at the wrong time - and end up in the hospital. It’s not because they’re careless. It’s because the labels are confusing. If you’ve ever stared at a pill bottle wondering what "XR" or "SR" means, or why your doctor said not to chew it, you’re not alone. Understanding how to read directions for extended-release medications isn’t just helpful - it’s life-saving.
What Extended-Release Means (And Why It Matters)
Extended-release medications are designed to release medicine slowly over hours, not all at once. This keeps your blood levels steady, so you don’t get spikes or crashes. Instead of taking a pill three or four times a day, you take it once or twice. Sounds simple, right? But here’s the catch: if you break, crush, or chew these pills, you’re not just changing how they work - you’re risking an overdose.Take OxyContin, for example. A single 60mg extended-release tablet is meant to release its painkiller over 12 hours. Crush it, and you get all 60mg at once - enough to stop breathing. That’s not hypothetical. The FDA recorded over 1,200 adverse events between 2018 and 2022 from people mishandling extended-release opioids alone.
Same goes for ADHD meds like Adderall XR. Take it at night thinking it’ll last all day? You’ll be wide awake for 36 hours. Take it with food when it says to take it on an empty stomach? It won’t work right. The label isn’t just advice - it’s science.
How to Spot Extended-Release Medications on the Label
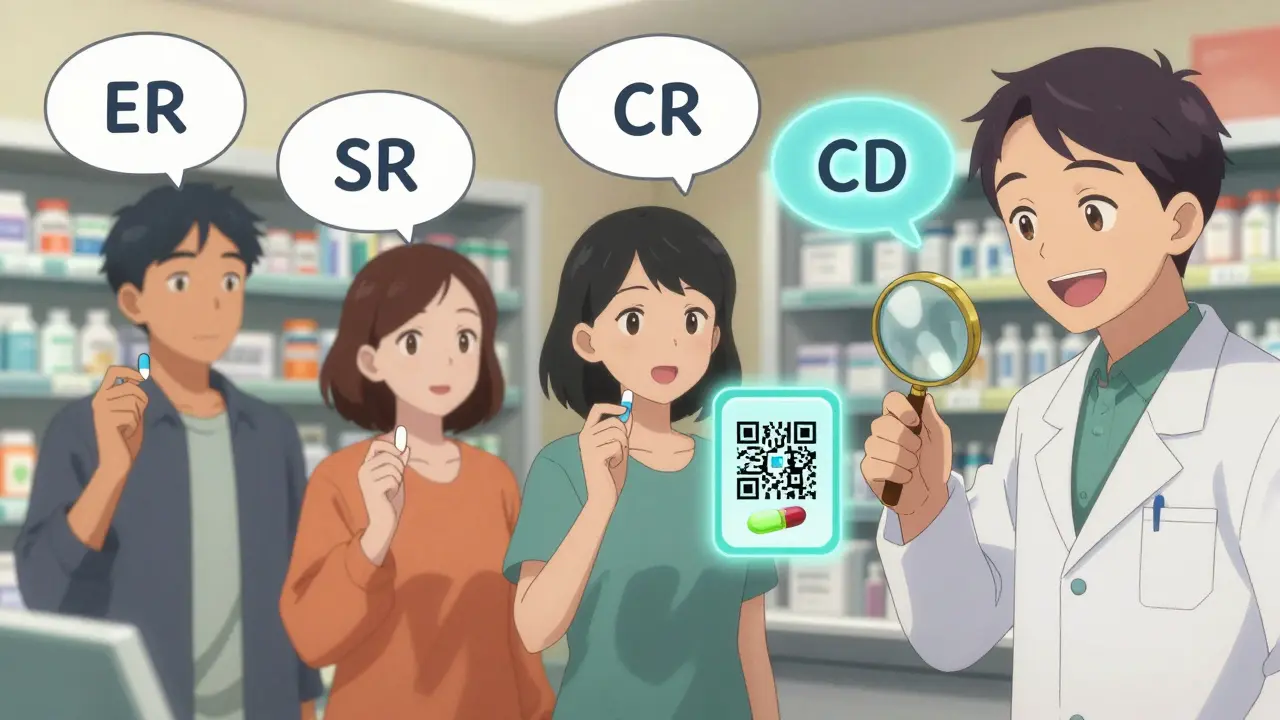
The first thing you need to do is look for the code. It’s usually right after the drug name. Here are the most common ones:- ER - Extended Release
- XR - eXtended Release
- SR - Sustained Release
- CR - Controlled Release
- CD - Continuous Delivery
- XT - Extended Release (brand-specific, like Cartia XT)
These aren’t just fancy letters. They’re warnings in disguise. If you see "metoprolol XR 25 mg" or "diltiazem CD 120 mg," you’re holding a modified-release pill. Never assume it’s the same as the regular version.
Also look for phrases like "24-hour extended release" or "12-hour sustained release." The FDA requires these timeframes to be clearly stated. A pill labeled "24-hour" isn’t the same as one labeled "12-hour," even if they have the same active ingredient. That’s why you can’t swap one brand for another without checking with your pharmacist.
The Three Main Ways Extended-Release Pills Work
Not all extended-release pills are made the same. How they release medicine depends on the technology used. Knowing the difference helps you understand why you can’t mess with them.- Matrix systems: The drug is mixed into a polymer (like a sponge soaked in medicine). As the pill moves through your gut, the polymer slowly breaks down, releasing the drug. Think of it like a sugar cube dissolving in tea - but over hours, not minutes.
- Coating techniques: The pill has a thin plastic-like shell that dissolves slowly. Some pills have multiple layers, each dissolving at a different time. Crushing it removes the coating and dumps the whole dose at once.
- Encapsulation: The medicine is sealed in a tiny capsule that opens at a set time. Some are designed to release in the intestine, not the stomach. Chew or open these, and you ruin the timing.
Here’s the kicker: two pills with the same name and same "XR" label might use totally different methods. Concerta (for ADHD) uses an osmotic pump - a tiny hole in the pill that pushes the drug out over 12 hours. A generic "XR" methylphenidate might use a matrix system. They’re not interchangeable. Your body absorbs them differently.
What to Look for on the Drug Facts Panel (OTC) or Prescription Label
Whether it’s a prescription or over-the-counter (like Aleve PM), the label has to follow FDA rules. Here’s what to check:- Drug Name and Strength: Always check the full name. "Metoprolol succinate ER" is different from "metoprolol tartrate." The suffix matters.
- Directions: "Take once daily in the morning" isn’t a suggestion. Some extended-release meds need to be taken with food. Others must be taken on an empty stomach. Niaspan (niacin XR) is taken at bedtime to reduce flushing. Get this wrong, and side effects get worse.
- Warnings: This section will say, "Do not crush, chew, or split." Sometimes it’s bold. Sometimes it’s in a box. Never ignore it. If it’s not there, ask your pharmacist. Some older labels still miss this.
- Inactive Ingredients: These are the fillers and coatings. Some people are allergic to dyes or lactose. If you have sensitivities, check this list. The FDA keeps a database of approved inactive ingredients - your pharmacist can look it up.
For OTC meds, look for the "Drug Facts" panel - it’s required by law since 2017. If you’re reading a tiny print label on a box with no clear section headings, it’s probably not FDA-compliant. Don’t trust it.
Why Timing and Food Matter More Than You Think
Extended-release meds aren’t just about how often you take them - it’s about when. Some are designed to work best with your body’s natural rhythms.For example:
- Cardizem CD (diltiazem): Best taken in the morning to control blood pressure during peak activity hours.
- Metformin XR: Often taken with dinner to reduce stomach upset and help control overnight blood sugar spikes.
- Gilenya (fingolimod): First dose must be taken at a clinic because it can slow your heart rate. That’s not on the bottle - it’s in the Medication Guide.
Food can change how fast the pill releases. Some extended-release drugs need fat to absorb properly. Others can’t handle it. Always follow the label. If it says "take on an empty stomach," wait two hours after eating. If it says "with food," have a snack. Don’t guess.
The Biggest Mistakes People Make
Based on real reports from pharmacists and the FDA, here are the top errors:- Crushing or splitting pills: Done by 1 in 5 patients who don’t realize it’s dangerous. Even pills with a score line aren’t always safe to split - only if the label says so.
- Swapping brands: Taking a generic "XR" version because it’s cheaper, without checking if it’s the same release system. Three different 24-hour diltiazem products exist - none are interchangeable.
- Taking at night when it should be morning: Especially common with stimulants like Adderall XR or non-stimulants like Strattera. Leads to insomnia, anxiety, or even heart palpitations.
- Ignoring the Medication Guide: For high-risk drugs (opioids, antidepressants, blood thinners), the pharmacy is legally required to give you a separate printed guide. Most people throw it away. Read it.
One pharmacist in Melbourne told me they see 2-3 cases a month of patients who crushed their extended-release pills. One man took his 30mg extended-release oxycodone and snorted it after splitting it - he ended up in intensive care. He didn’t think it was a big deal because "it’s just a pill."
What to Do If You’re Unsure
You don’t have to figure this out alone. Here’s what to do:- Ask your pharmacist: They’re trained to explain this stuff. Say: "Can you show me how this pill works?" or "Is it safe to split this?"
- Use the teach-back method: After they explain, repeat it back in your own words. "So you’re saying I take this once a day, in the morning, and I can’t crush it?" If they nod, you got it right.
- Scan the QR code: Newer labels have QR codes that link to short videos showing how to take the pill. It’s not gimmick - it’s FDA-approved patient education.
- Keep a list: Write down the name, dose, frequency, and special instructions for each extended-release med you take. Keep it in your wallet or phone.
If you’re over 65, you’re at higher risk of misunderstanding labels. A 2022 FDA study found 42% of older adults thought "extended release" just meant "lasts longer," not "don’t break it." Don’t assume you know what it means - ask.
What’s Changing in 2025
The system is getting better - slowly. Starting January 2024, the FDA requires all new extended-release prescriptions to have high-contrast "DO NOT CRUSH" warnings. By 2025, electronic health records must show "24-hour extended release" instead of just "ER" to avoid confusion.Some companies are testing pills that change color when crushed. Others are using smart packaging that alerts your phone if you open the bottle too early. In the next five years, nearly half of all new drugs will be extended-release. That means understanding this isn’t optional anymore.
It’s not about being perfect. It’s about being careful. One wrong move with an extended-release pill can change your life - or end it. Don’t let confusion be the reason.
Can I split an extended-release pill if it has a score line?
Not always. Just because a pill has a score line doesn’t mean it’s safe to split. Some extended-release pills use coatings or matrix systems that are ruined by splitting, causing the full dose to release at once. Only split if the label or your pharmacist explicitly says it’s safe. For example, some versions of metoprolol succinate ER are approved to be split, but others aren’t. Always check.
What’s the difference between ER, XR, and SR?
In practice, ER, XR, and SR all mean the drug is released slowly over time. But the exact mechanism can vary. ER and XR are often used interchangeably, while SR sometimes implies a more gradual release. The key isn’t the acronym - it’s the timing and instructions on the label. A "24-hour ER" and a "24-hour XR" might be identical, but a "12-hour SR" is not the same as either. Always read the full description.
Why can’t I take my extended-release pill with grapefruit juice?
Grapefruit juice can interfere with how your body breaks down certain medications - especially those processed by the liver enzyme CYP3A4. Many extended-release drugs, like some statins, blood pressure meds, and anti-anxiety pills, are affected. This can cause the drug to build up to dangerous levels in your blood. Even if the label doesn’t mention grapefruit, it’s safest to avoid it unless your pharmacist says it’s okay.
Are generic extended-release pills as safe as brand-name ones?
They’re required to have the same active ingredient and similar absorption rates, but not always the same release mechanism. For example, three different 24-hour diltiazem generics exist - none are interchangeable with each other or the brand-name version. The FDA doesn’t require generics to match the exact technology used in the original. Always ask your pharmacist if the generic you’re getting is the same release type as your original prescription.
What should I do if I accidentally crush or chew an extended-release pill?
Call your pharmacist or poison control immediately. Don’t wait for symptoms. Even if you feel fine, the full dose may still be entering your system. For opioids or heart meds, this can be life-threatening within minutes. Keep the poison control number (13 11 26 in Australia) saved in your phone. If you’re with someone who’s had an accident, stay with them and monitor their breathing until help arrives.
If you take extended-release medications, make this your rule: Read the label. Ask the pharmacist. Never guess. Your body doesn’t work on assumptions - and neither should you.


Let me guess - you crushed your Adderall XR because you "needed to focus" and now you’re wondering why your heart feels like it’s trying to escape your chest. Classic. The label isn’t a suggestion, it’s a fucking warning. If you can’t read "DO NOT CRUSH," maybe you shouldn’t be taking pills at all.
Also, "XR" isn’t a brand name, it’s a pharmacokinetic design. Stop treating your meds like a TikTok hack.