When your pharmacist hands you a pill that looks nothing like the one you’ve been taking for years, it’s normal to pause. Is this the same medicine? Will it even work? You’re not alone. Millions of people in Australia and around the world switch from brand-name drugs to generics every year - mostly because it saves money. But what really happens when you make the switch? And should you be worried?
Generics aren’t cheap copies - they’re exact copies, legally
The biggest myth about generic drugs is that they’re inferior. They’re not. In Australia, the Therapeutic Goods Administration (TGA) requires generics to meet the same strict standards as brand-name drugs. That means the same active ingredient, same strength, same way it works in your body. The TGA doesn’t allow a generic drug to be sold unless it proves it delivers the exact same amount of medicine into your bloodstream at the same speed as the original. That’s called bioequivalence.
So why do they look different? Because trademark laws force generic makers to change the shape, color, or size of the pill. A blue oval from one company might be a white circle from another. That’s just for branding - not for effectiveness. Your body doesn’t care what the pill looks like. It only cares about the chemical inside.
And here’s the real kicker: most generics are made in the same factories as brand-name drugs. The TGA inspects them all the same way. A 2023 review by the U.S. FDA found that over 90% of all prescriptions filled in the U.S. were for generics - and those same generics are used in Australia, Canada, and the UK under similar rules.
Cost savings are real - and huge
Let’s talk numbers. A 30-day supply of brand-name Lipitor (atorvastatin) might cost you $120 out-of-pocket. The generic version? Around $12. That’s not a typo. Same drug. Same results. Same safety profile. The difference? $108 saved per month.
That’s why insurance companies push generics. In Australia, PBS (Pharmaceutical Benefits Scheme) listings heavily favor generics. If you’re on a concession card, your co-payment for a generic might be under $7. For brand-name versions, you could pay over $30 - if it’s even covered.
And it’s not just about pills. A 2019 study showed that patients who started on brand-name drugs were 2.6 times more likely to quit taking them because of cost. That’s not just inconvenient - it’s dangerous. Skipping heart meds, diabetes drugs, or thyroid pills because they’re too expensive leads to hospital visits, complications, and worse outcomes. Generics keep people on their meds.
When switching might cause problems - and who should be careful
For most people, switching is smooth. But not for everyone. There’s a small group of medications where even tiny differences in how the drug is absorbed can matter. These are called narrow therapeutic index drugs. A little too much? Toxic. A little too little? Ineffective.
Examples include:
- Warfarin (blood thinner)
- Levothyroxine (thyroid hormone)
- Phenytoin, carbamazepine (anti-seizure meds)
- Lithium (mood stabilizer)
There are documented cases where patients switched from brand to generic levothyroxine and saw their TSH levels jump from 2.5 to 8.7 - meaning their thyroid stopped working properly. Others on warfarin had abnormal INR readings after a generic switch, increasing stroke or bleeding risk.
That doesn’t mean generics are bad. It means these drugs need close monitoring. If you’re on one of these, talk to your doctor before switching. Ask for a blood test 4-6 weeks after the switch to make sure your levels are still in range.
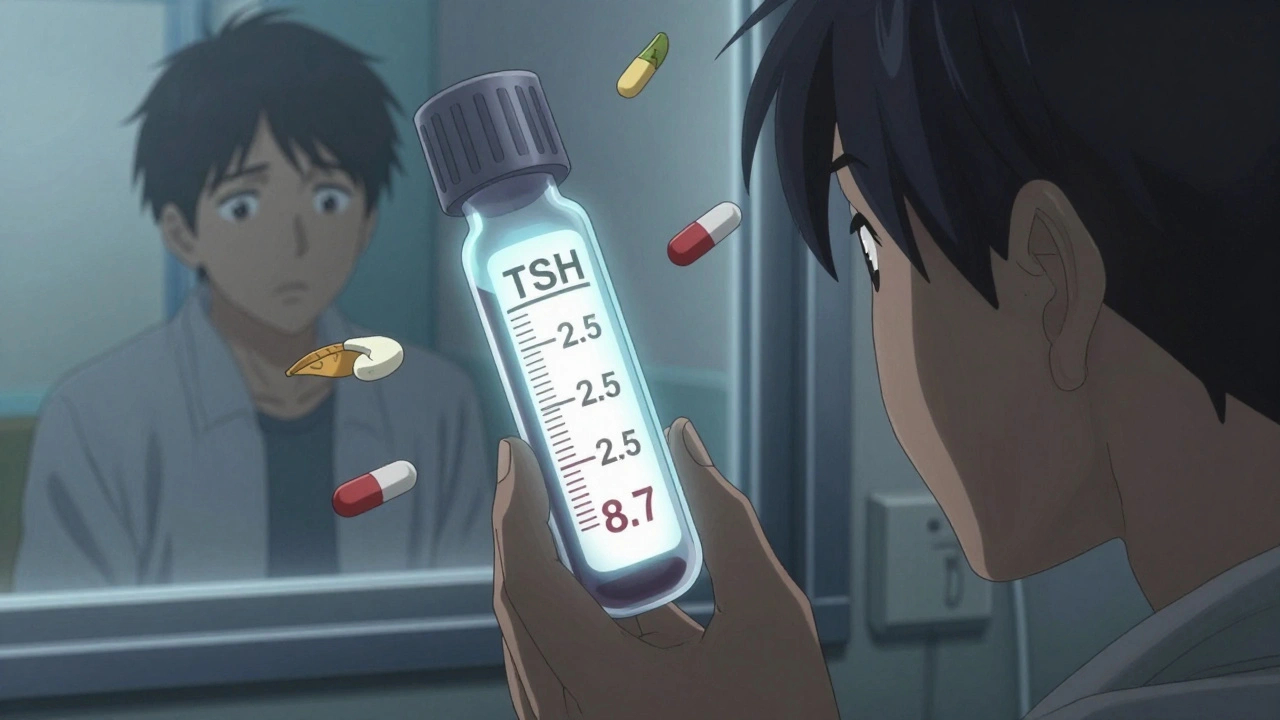
Why you might feel different - even if the drug is the same
Some people swear they feel worse on generics. Headaches. Fatigue. Mood swings. But the active ingredient hasn’t changed. So what’s going on?
It’s often the inactive ingredients. Generics use different fillers, dyes, or preservatives. For most people, these are harmless. But if you’re allergic to lactose, red dye, or certain coatings, you might react. One patient in Melbourne switched from a brand-name antidepressant to a generic and developed a rash - turns out the new version had a dye she was sensitive to. Her doctor switched her back, and the rash disappeared.
Also, psychology plays a role. If you believe generics are inferior, your brain might amplify minor side effects. That’s called the nocebo effect. One study in Massachusetts found that 63% of patients were worried about generics at first - but after three months, 82% said they were satisfied. Once they saw the results, their fears faded.
What to do when you get a new-looking pill
Pharmacies in Australia are allowed to switch your prescription to a generic unless your doctor writes “dispense as written.” That’s standard practice. But you should still know what’s happening.
Here’s what to do:
- Ask your pharmacist: “Is this a different brand or generic?” They’ll tell you.
- Check the label. It should list the active ingredient - that’s what matters.
- Compare the pill shape and color to your old one. If it’s different, that’s normal - unless your doctor said otherwise.
- Don’t stop taking it. Give it 2-4 weeks. If you feel off, call your doctor. Don’t assume it’s the drug.
- Keep a simple log: date switched, how you felt, any new symptoms. Bring it to your next appointment.
Some pharmacies now put stickers on bottles saying “This is a generic version of [brand name].” That helps. But not all do. So ask.

When you can ask to stick with the brand
You have rights. If your doctor thinks a brand-name drug is better for you - because of your history, sensitivity, or condition - they can write “Do Not Substitute” or “Dispense as Written” on your prescription. That legally blocks the pharmacist from switching it.
Common reasons doctors do this:
- You’ve had problems switching before (like seizures or unstable INR)
- You’re on a narrow therapeutic index drug and your levels are finely tuned
- You’ve had an allergic reaction to a generic’s filler
- You’re pregnant or breastfeeding and your doctor prefers the brand’s safety record
Don’t feel guilty asking. It’s your health. If your doctor says no, ask why. Sometimes it’s just habit. Other times, it’s based on real evidence.
What’s changing in 2025
More pharmacies are starting to use color-coded labels or QR codes on generic bottles that link to a simple page explaining the drug’s equivalence. It’s still early, but it’s a step toward reducing confusion.
Also, the TGA is pushing for more consistent pill appearances across generic brands - especially for high-risk drugs. Imagine if all levothyroxine generics looked the same, no matter who made them. That would cut down on mistakes.
And insurance? They’re tightening the rules. More plans now require you to try the generic first. If it doesn’t work, you can appeal. But you need documentation - your doctor’s note, lab results, symptom log.
Bottom line: It’s usually safe - but stay alert
For 9 out of 10 people, switching to a generic drug is a smart, safe, and affordable move. You’ll save hundreds - even thousands - a year. And you’ll get the same clinical results.
But for the 1 in 10 - especially those on blood thinners, thyroid meds, or seizure drugs - switching needs care. Monitor your body. Talk to your doctor. Don’t assume everything is fine just because the label says “generic.”
Generics aren’t a compromise. They’re a smart choice - if you’re informed. The goal isn’t to scare you off generics. It’s to help you use them wisely.

Let me guess - the pharmaceutical companies paid the TGA to say generics are ‘just as good’ - same factories? LOL. You think they’d let a Chinese plant make the same pill as Pfizer if it wasn’t for the $$$? They’re using cheaper fillers that mess with your gut biome. I’ve seen people on generics get weird rashes, brain fog, even panic attacks. They don’t test for long-term effects because they don’t want you to know. Wake up.